结晶氮链自由基阴离子
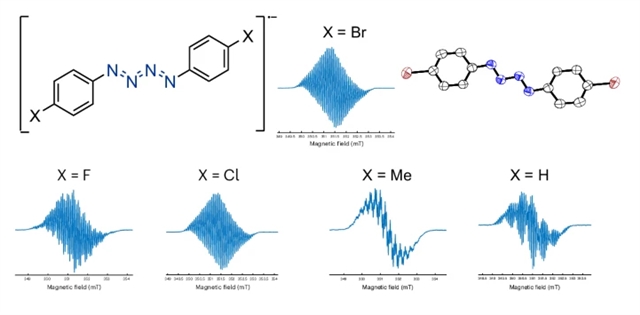
近日,英国牛津大学Meera Mehta团队研究了结晶氮链自由基阴离子。2026年2月10日出版的《自然-化学》杂志发表了这项成果。
长链氮离子及自由基([Nn]x+/[Nn]x-,n>3)在地球电离层及其他行星天体的强辐射环境中天然存在。然而,由于释放N2的强热力学驱动力,这类分子在常温常压下极其活泼,通常只能在极端条件(例如10 GPa至>200 GPa的超高压)下进行研究。
研究组报道了在常温常压下分离的五例金属非支撑型{ N4}·-单元分子,其中一例衍生物在固态下表现出长达数周的显著稳定性。光谱学、晶体学及计算研究揭示了{ N4}·-链的成键本质。反应性研究表明,该氮链可裂解为N1和N3片段,并可作为氮烯自由基负离子的供体——这一发现预示此类分子有望用作可存储型氮基团转移试剂。
附:英文原文
Title: Crystalline nitrogen chain radical anions
Author: Lister-Roberts, Reece, Galano, Daniel, van IJzendoorn, Bono, Whitehead, George F. S., Brookfield, Adam, Bowen, Alice M., Kaltsoyannis, Nikolas, Mehta, Meera
Issue&Volume: 2026-02-10
Abstract: Long-chain nitrogen ions and radicals ([Nn]x+/[Nn]x, n>3) are naturally occurring under the intense radiative conditions of the Earth’s ionosphere and those of other planetary bodies. However, the strong thermodynamic driving force to lose N2 renders these types of molecule extremely reactive under ambient conditions such that they can typically be studied only under extreme conditions, for example, at ultrahigh pressures (10GPa to >200GPa). Here we report the isolation of a series of five molecules featuring metal unsupported {N4} units under ambient conditions, with one derivative demonstrating remarkable multi-week long persistence in the solid state. Spectroscopic, crystallographic and computational studies provide insight into the bonding across the {N4} chain. Reactivity studies reveal that the chain can cleave into N1 and N3 fragments, and can act as a source of nitrene radical anions, an observation that such molecules could act as storable nitrogen group transfer reagents.
DOI: 10.1038/s41557-025-02040-2
Source: https://www.nature.com/articles/s41557-025-02040-2



